Neurological diseases such as epilepsy and Alzheimer’s inflect a heavy toll on human population,
both in terms of health and economic impact. The lack of understanding of the disease pathogeneses hinders the efforts to
develop efficient therapeutics. We use computational techniques to gain deep insight into the function and dysfunction of biological systems, particulalry the pathogeneses of
neuronal disorders. The following projects are currently underway in our Lab.
Neuronal Microenviornment
In in various brain states the excitability of neuronal networks not only depends on the nonlinear interaction between excitatory and inhibitory neuronal subtypes but also a variety of metabolic processes such as potassium concentration gradients and local oxygen availability. Our motivation is to develop next generation biophysical models that will account for the metabolic variables, such as potassium, sodium, chloride, calcium and oxygen concentrations, glucose supply, and pH levels along with the electrical component (Figure 1). An essential component of these models are the glial network and vasculature surrounding the neurons. These models will enable us to study the role of neuronal microenvironment and metabolic variables in normal brains function, epileptic seizures, spreading depression, hypoxia, and hypoxia-induced seizures.
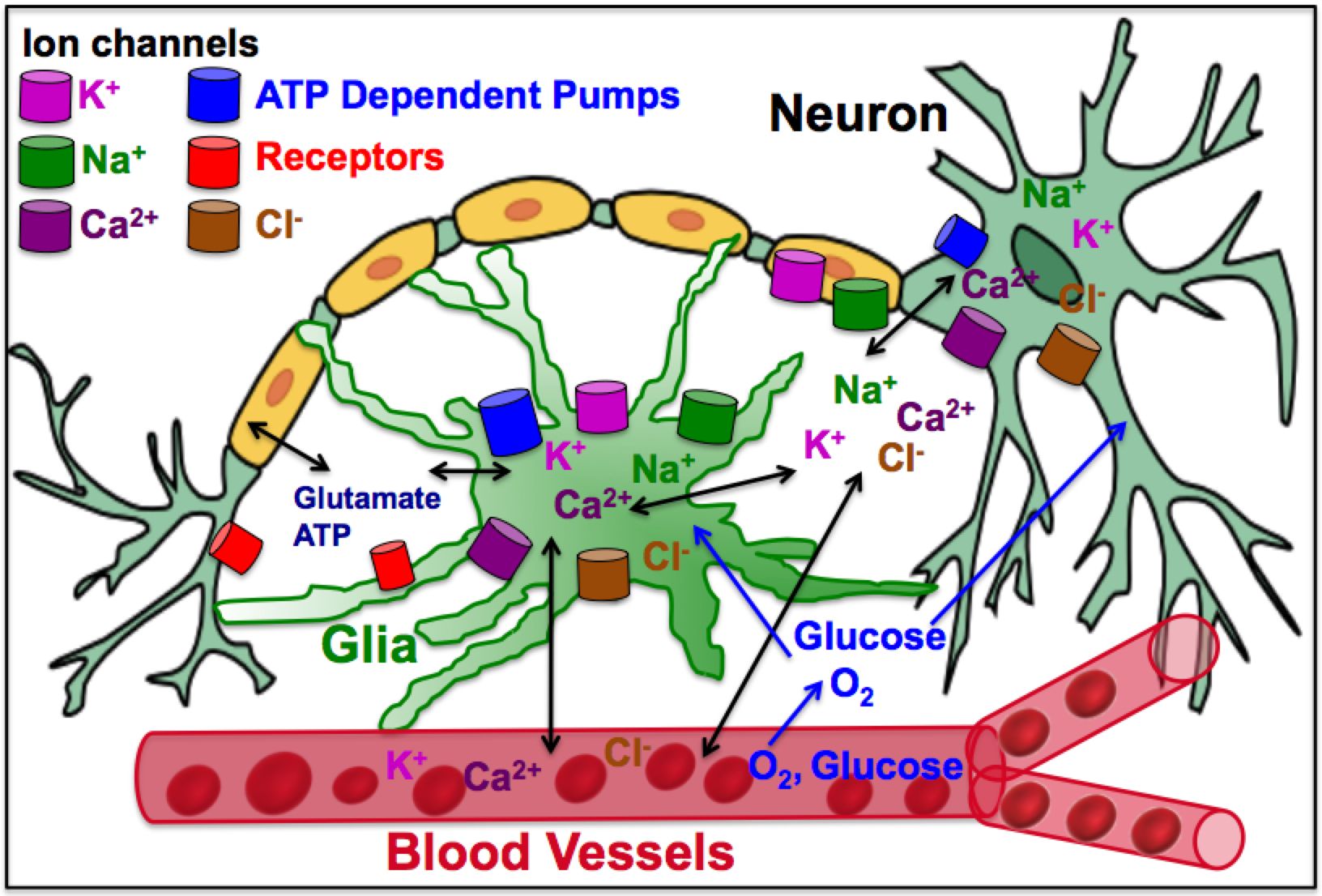
Figure 1: Neuronal microenvironment affecting neuronal function.
Calcium Signaling in Neurodegenerative Diseases
Overwhelming evidence suggests a key role of calcium signaling dysfunction in Alzheimer's and other age-related diseases. Calcium signaling dysfunction accounts for the early cognitive and memory impairments as well as the progressive cell death during neurodegenerative diseases. However, the molecular pathways leading to the observed calcium dysfunction are not well understood. We develop computational models to pinpoint the signaling pathways that are upstream in the calcium cascade and to understand their role in cell bioenergetics and apoptosis (Figure 2). Once in place, this computational framework will be easily adoptable for other diseases such as Parkinson's, Huntingtons, Amyotrophic Lateral Sclerosis, Spinocerebellar ataxias, and Sarcopenia.
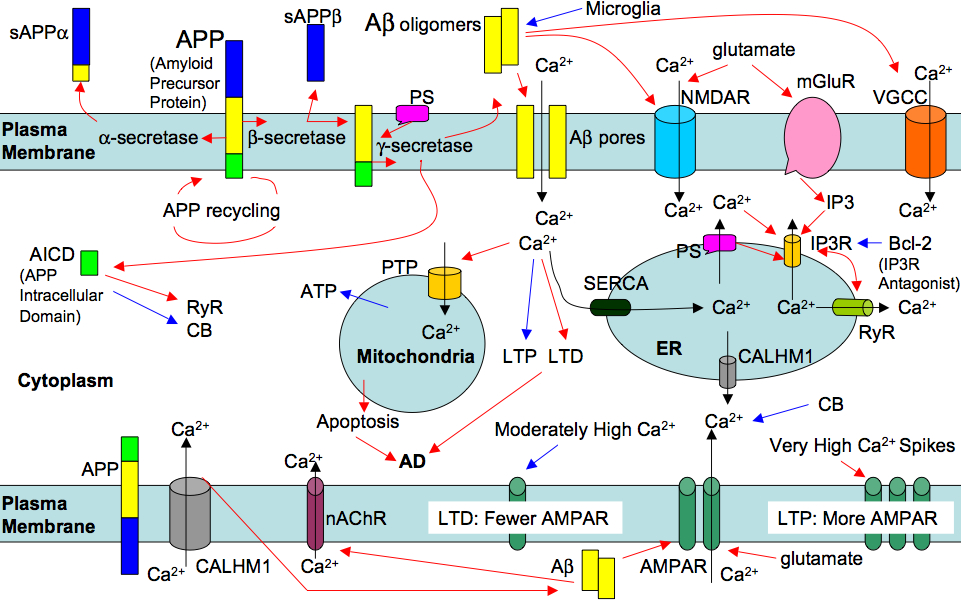
Figure 2: Alzheimer's associated peptides Amyloid beta and presenilin proteins disrupt many pathways involved in calcium signaling leading to higher calcium concentration in the cytoplasm. The higher cytosolic calcium concentration potentially leads to mitochondrial dyfunction and apoptosis. Red/Blue/Black arrows represent upregulation/downregulation/calcium flux.
Application of Control Theory in Biology
Existing experimental techniques can measure only a few of the many variables controlling the dynamics of biological systems. However, recent advances in nonlinear control theory offer a paradigm shifting improvement in our ability to assimilate, predict, and control spatiotemporal biological systems. We continue to use cutting edge state reconstruction techniques such as Kalman filter to estimate the experimentally inaccessible biological variables from as few as one experimentally observed variable (Figure 3). We hope to implement these tools for predicting and controlling of biological systems in future.
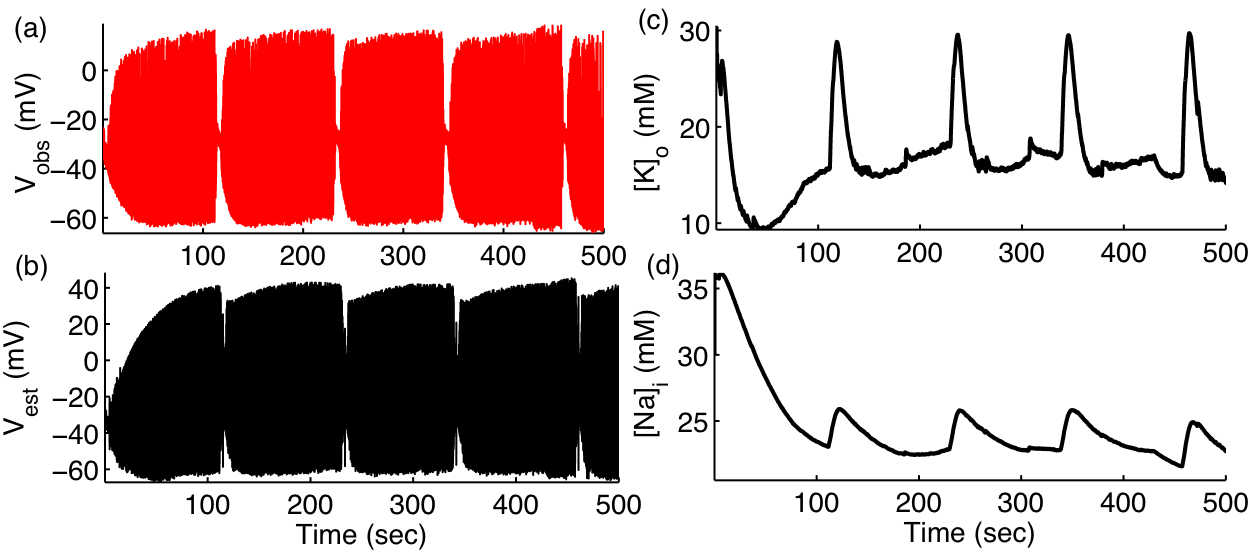
Figure 3: Estimating full neuronal dynamics (Black) only from membrane potential measurements (Red) during spontaneous seizures.