Current Projects
Mechanisms of Amyloid Fibril Self-assembly
The main focus of our current research is on the molecular mechanisms regulating self-assembly of proteins into amyloid fibrils. Amyloid fibrils are long, nonbranching protein fibrils characterized by their cross-beta sheet architecture. These fibrils are the molecular hallmark of a multitude of human disorders that includes many neurodegenerative diseases such as Alzheimer's and Parkinson's disease, prion diseases, but also type II diabetes, light chain and lysozyme amyloidoses and, potentially, even glaucoma. At the same time, there are several examples that amyloid fibril formation is associated with functional biological activity in bacteria, yeast and even mammals. In fact, there is increasing evidence that the backbones of polypeptide chains, which are the polymers that peptides and proteins are made of, have the intrinsic propensity towards forming the intermolecular hydrogen bonds that are the structural characteristic of amyloid fibrils. Yet, many aspects of the mechanisms that induce proteins and peptides to form amyloid fibrils in vitro and in vivo remain poorly understood.
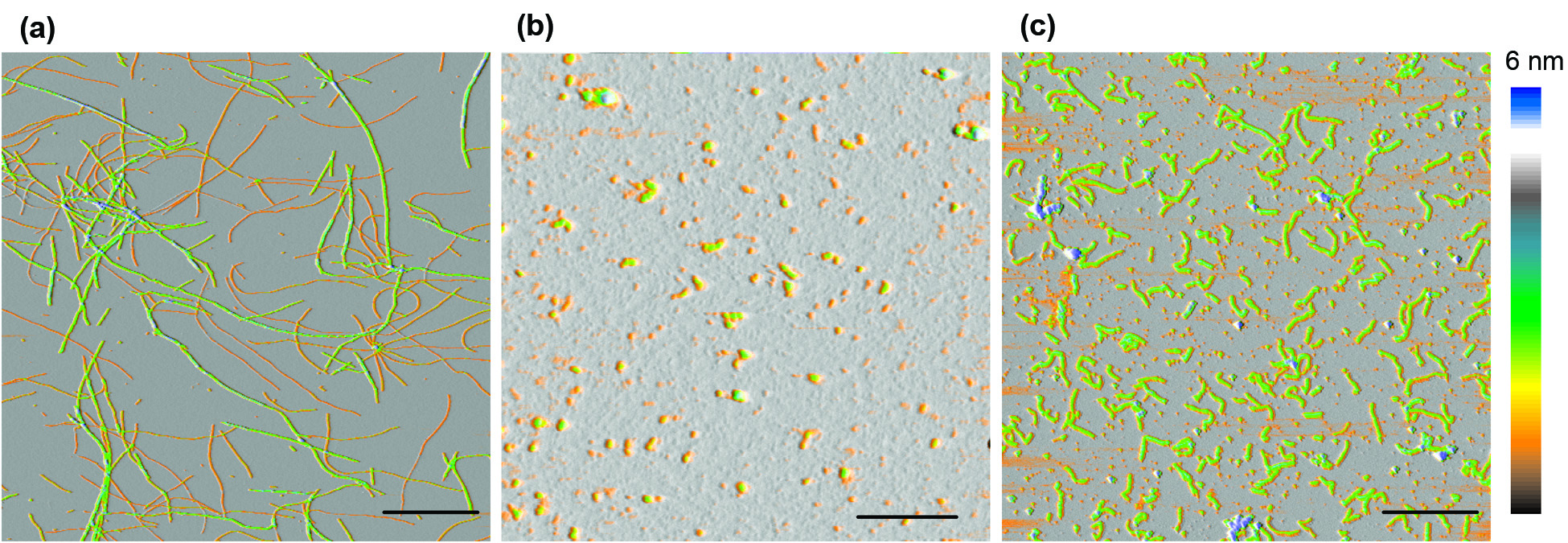
Oligomeric Intermediates and Assembly Pathways
One striking features of amyloid assembly is the frequent presence of small globular oligomers (O) and curvilinear fibrils (CF) that are distinct from the late-stage rigid filaments and fibrils (RF). We set out to determine the assembly kinetics, as well as their tinctorial, morphological and structural characteristic of these various aggregate species during lysozyme amyloid growth. We assembled evidence that oligomers and curvilinear fibrils are forming along the same assembly pathway, which is distinct from the nucleated polymerization pathway for rigid fibril formation. (Hill et al, Biophys. J. 2009, Hill et al. Plos One 2011, Foley et al., 2013).
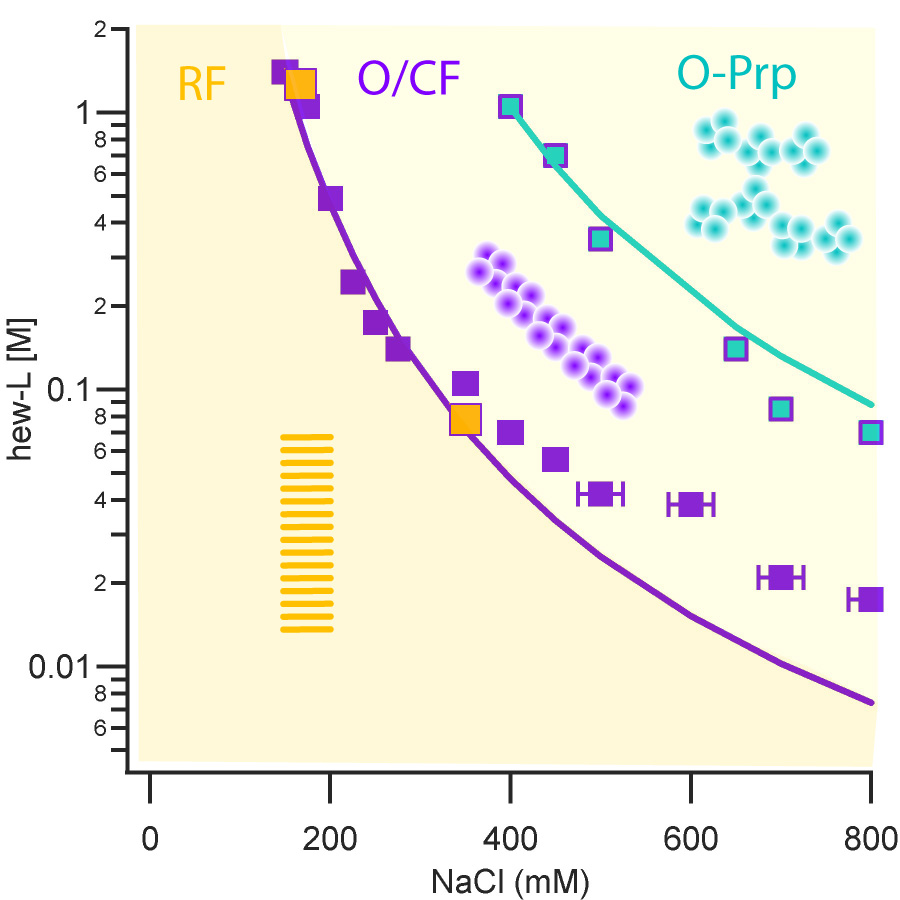
Metastable Oligomers and “Critical Oligomer Concentration”
Based on the above observations, we mapped out the solution conditions (salt, protein concentrations) that induced the transition from pure nucleated polymerization of rigid fibrils to the onset of oligomer/curvilinear fibril formation. Our results delineate a clear transition boundary which we called “critical oligomer concentration” or COC due to its similarity to critical micelle concentrations in surfactant systems. In collaboration with my collaborator Jeremy Schmit at Kansas State, we could replicate this COC by accounting for the salt- and protein-dependence of the COC by accounting for the free energy cost of confining charged monomers to the volume of an oligomer. We further showed that oligomers and curvilinear fibrils were metastable agains the growth of rigid fibrils seeded above the COC (Miti et al, Biomacromolecules 2015).
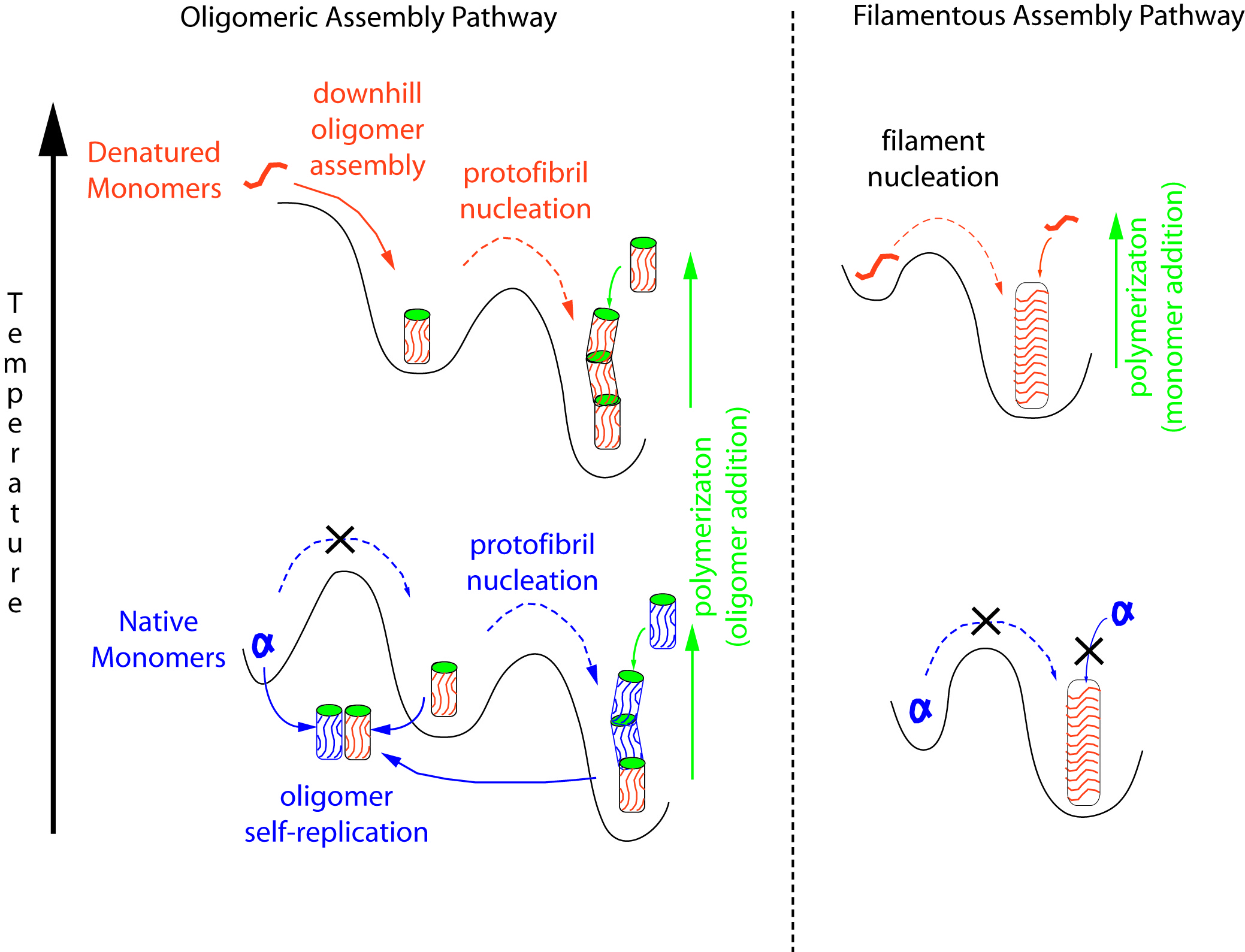
Ongoing Research Projects
Charge Repulision and its Effects on Amyloid Formation
The free energy of solutes in solution implies that solutes will only phase separate if they have net attractive interactions so that they can overcome the dispersive effects of entropy. Yet, under the low salt and acidic pH conditions promoting in vitro amyloid growth, the potential of net force among lysozyme monomers is typically repulsive. The strength of this net repulsion correlates with the type of amyloid aggregates that will form: oligomer and protofibril assembly prevails at weak repulsion while filament formation emerges as charge repulsion increases. (Hill et al. (2011) PLoS One 6:e18171) We are testing our hypotheses that weak net repulsion still permits favorable intermolecular interactions among those partially denatured conformations most prone to fibril formation while keeping all other native and partially unfolded conformations in solution. By varying the net charge of lysozyme and ionic charge screening, we are currently testing this correlation over a wide range of solution conditions
Model Systems of Amyloid Fibril Assembly
We are investigating the assembly behavior of simple polyamino acids that have previously been shown to form amyloid fibril formation. (Fändrich et al. (2002) EMBO J. 21:5682) We are using these simple models of alpha-helical polymers to investigate whether fibril formation precedes, coincides with or results from the structural remodeling of the polypeptide monomers from their alpha-helical to beta-sheet conformation.
Cellular Toxicity of Different Amyloid Intermediates
Lysozyme amyloid assembly can proceed along two distinct assembly pathways that generate distinctly different intermediates and late-stage products. (Hill et al. (2011) PLoS One 6:e18171) In the "oligomeric" pathway, the first assembly step is the formation of globular oligomers of a very narrow size range (~ 8 monomers) that subsequently polymerize into curvilinear polymers (protofibrils). Along the "monomeric filament" pathway long (multiple m) and rigid filaments nucleate after a long lag phase. These filaments originally have monomeric cross-sections and tend to fragment and can bundle into thicker fibrils. In collaboration with my colleague Dr. Chad Dickey at the Byrd Alzheimer Institute, we are testing which of these different aggregate populations are most deleterious to cells.